My group use biophysical techniques to decipher the molecular details of large protein complexes involved in bacterial pathogenesis and antibiotic resistance. In particular, we are exploiting high-resolution cryo-electron microscopy (cryo-EM) for studying potential new antibiotics targets. In the past three years, cryo-EM has revolutionized our capacity to look at biological molecules at the level of individual atoms.
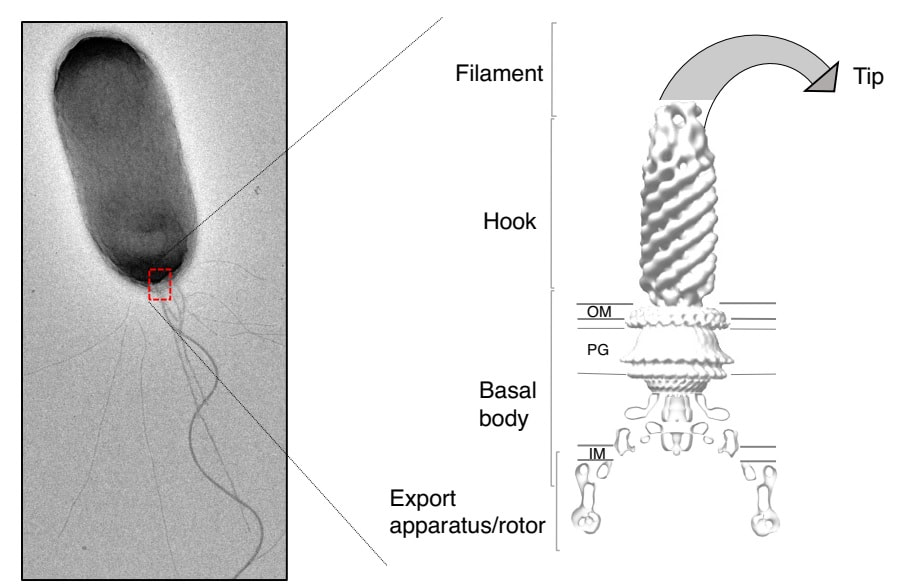
Specifically, we are studying the bacterial flagellum, a long rotating filament that acts as a propeller, allowing them to move and adhere on the surface of target cells. For pathogenic bacteria (such as E. coli, Salmonella and Yersinia), flagella can aid the spread of disease, and helps them attach to medical devices such as catheters. As a consequence, understanding the bacterial flagellum at the molecular level could have numerous medical implications, and disrupting its rotation and adherence properties could significantly reduce infection in a clinical setting. Because of its size and complexity, characterizing the flagellum at the molecular level was previously impossible. But with the recent advances of cryo-EM, we can now study this complex with unprecedented detail.
Read more about Julien's research at: www.bergeronlab.com
Follow the official hashtag on twitter: #BSW18